Ralph Pearson introduced the Hard Soft [Lewis] Acid Base (HSAB) principle in the early nineteen sixties, and in doing so attempted to unify inorganic and organic reaction chemistry. The impact of the new idea was immediate, however, over the years the HSAB principle has rather fallen by the wayside while other approaches developed at the same time, such as frontier molecular orbital (FMO) theory and molecular mechanics, have flourished.
Introduction
The Irving-Williams stability series (1953) pointed out that for a given ligand the stability of dipositive metal ion complexes increases:
Ba2+ < Sr2+ < Ca2+ < Mg2+ < Mn2+ < Fe2+ < Co2+ < Ni2+ < Cu2+ < Zn2+
It was also known that certain ligands formed their most stable complexes with metal ions like Al3+, Ti4+ and Co3+ while others formed stable complexes with Ag+, Hg2+ and Pt2+.
In 1958 Ahrland et al. Classified metal cations as Type A and Type B, where:
Type A metal cations included:
- Alkali metal cations: Li+ to Cs+
- Alkaline earth metal cations: Be2+ to Ba2+
- Lighter transition metal cations in higher oxidation states: Ti4+, Cr3+, Fe3+, Co3+
- The proton, H+
Type B metal cations include:
- Heavier transition metal cations in lower oxidation states: Cu+, Ag+, Cd2+, Hg+, Ni2+, Pd2+, Pt2+.
Ligands were classified as Type A or Type B depending upon whether they formed more stable complexes with Type A or Type B metals, from here:
Tendency to Complex
with Type A Metals
|
Tendency to Complex
with Type B Metals
|
N >> P > As > Sb > Bi
O >> S > Se > Te
F >> Cl > Br > I
|
N << P > As > Sb > Bi
O << S ~ Se ~ Te
F < Cl < Br << I
|
From this analysis, a principle can be derived:
Type A metals prefer to bind to Type A ligands
and
Type B metals prefer to bind to Type B ligands
These empirical (experimentally derived) rules tell us that Type A metals are more likely to form oxides, carbonates, nitrides and fluorides, while Type B metals are more likely to form phosphides, sulfides and selinides. This type of analysis is of great economic importance because some metals are found in nature as sulfide ores: PbS, CdS, NiS, etc., while other are found as carbonates: MgCO3 and CaCO3 and others as oxides: Fe2O3 and TiO2.
This approach has been very successful developed in recent years by Bruce Railsback with hisexcellent and highly recommended "Earth Scientist's Periodic Table", here.
- The Railsback analysis uses contours of behaviour superimposed upon the Mendeleev periodic table. (As Bruce told me in a personal communication: "Earth scientists love contours...").
- See the paper: A Synthesis of Systematic Mineralogy by Bruce Railsback that develops this analysis.
Pearson's Hard Soft [Lewis] Acid Base Principle
In the nineteen sixties, Ralph Pearson developed the Type A and and Type B logic by explaining the differential complexation behaviour of cations and ligands in terms of electron pair donating Lewis bases and electron pair accepting Lewis acids:
Lewis acid + Lewis base  Lewis acid/base complex Lewis acid/base complex
Pearson classified Lewis acids and Lewis bases as hard, borderline or soft.
According to Pearson's hard soft [Lewis] acid base (HSAB) principle:
Hard [Lewis] acids prefer to bind to hard [Lewis] bases
and
Soft [Lewis] acids prefer to bind to soft [Lewis] bases
At first sight, HSAB analysis seems rather similar to the Type A and Type B system. However, Pearson classified a very wide range of atoms, ions, molecules and molecular ions as hard, borderline or soft Lewis acids or Lewis bases, moving the analysis from traditional metal/ligand inorganic chemistry into the realm of organic chemistry.
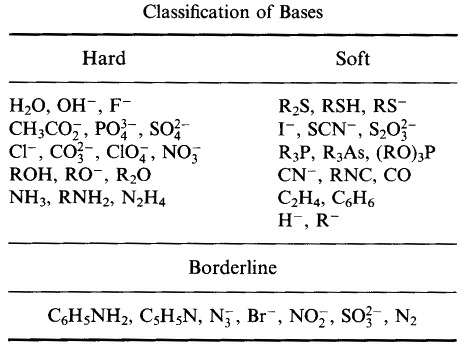
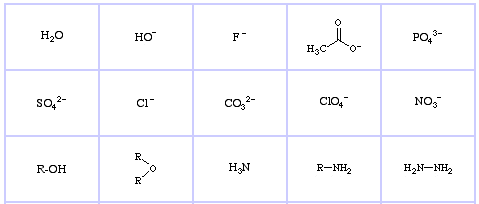
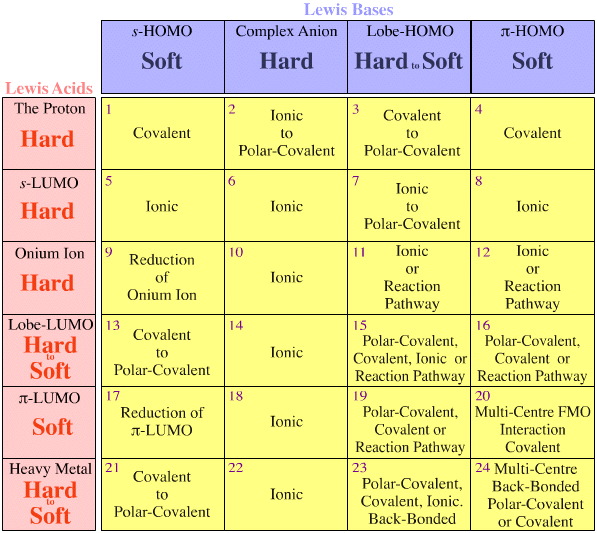
Pearson's HSAB Classification System, from here:
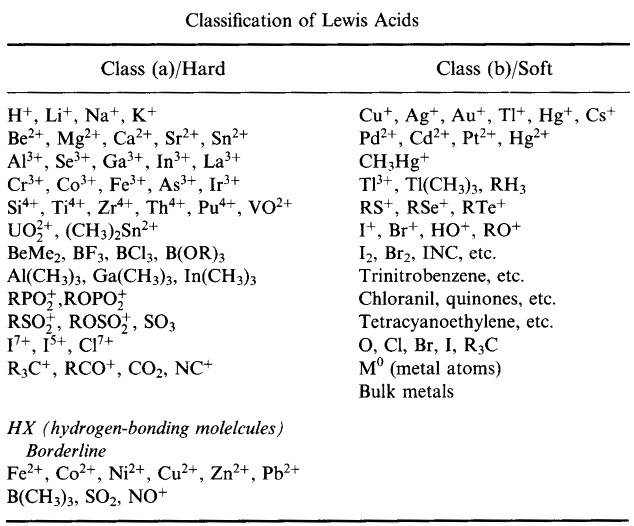
Pearson's Hard Lewis Acids (from the Chemical Thesaurus), here, and from the congeneric array database, here:
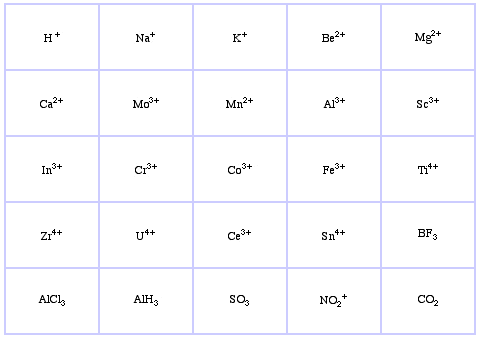
Pearson's Borderline Lewis Acids, here, and here:
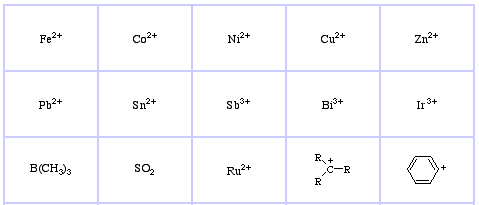
Pearson's Soft Lewis Acids, here, and here:
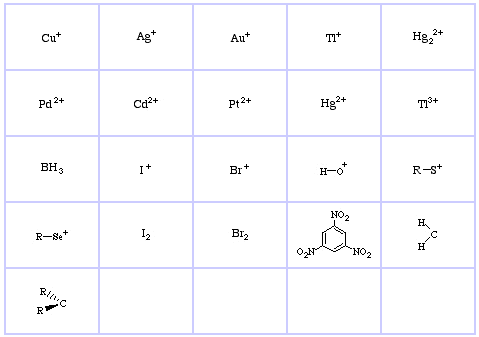
Pearson's Hard Lewis Bases (from The Chemical Thesaurus), here, and from the congeneric array database, here:
Pearson's Borderline Lewis Bases, here, and here:

Pearson's Soft Lewis Bases, here, and here:
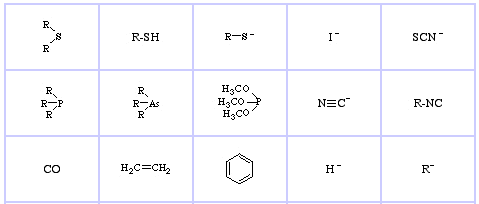
Klopman's FMO Analysis
In 1968, G. Klopman attempted to quantify Pearson's HSAB principle using frontier molecular orbital (FMO) theory, as discussed elsewhere in this web book, here, with this equation:
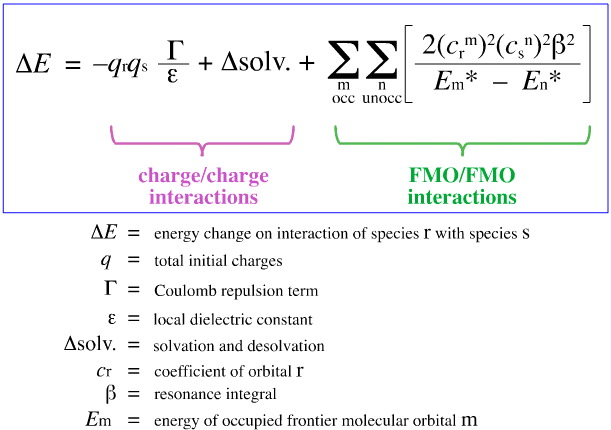
Klopman proposed that:
Hard [Lewis] acids bind to hard [Lewis] bases to give charge-controlled (ionic) complexes. Such interactions are dominated by the +/– charges on the Lewis acid and Lewis base species.
and
Soft [Lewis] acids bind to soft [Lewis] bases to give FMO-controlled (covalent) complexes. These interactions are dominated by the energies of the participating frontier molecular orbitals (FMO), the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO).
Read more elsewhere in the Chemogenesis web book, here, or look at Ian Fleming's Organic Chemistry and FMO theory here, where these ideas are developed at some length.
Using this analysis, the contributing aspects of charge-controlled and FMO-controlled Lewis acid/base complexation are separated and quantified, a crucial development.
Combining Pearson's and Klopman's Ideas
• Hard Lewis acids:• Atomic centres of small ionic radius
• High positive charge
• Species do not contain electron pairs in their valence shells
• Low electron affinity
• Likely to be strongly solvated
• High energy LUMO
• Soft Lewis acids:
• Large radius
• Low or partial δ+ positive charge
• Electron pairs in their valence shells
• Easy to polarise and oxidise
• Low energy LUMOs, but large magnitude LUMO coefficients
• Hard Lewis bases:
• Small, highly solvated, electronegative atomic centres: 3.0-4.0
• Species are weakly polarisable
• Difficult to oxidise
• High energy HOMO
• Soft Lewis bases:• Large atoms of intermediate electronegativity: 2.5-3.0
• Easy to polarise and oxidise
• Low energy HOMOs but large magnitude HOMO coefficients
• Borderline species have intermediate properties.
- There is a qualifier in Klopman's paper saying that it is not necessary for species to possess all properties.
The Ho Paper
Pearson suggested that hard-to-soft trends could be found amongst groups 15, 16 and 17 of the periodic table.
in 1975 the idea was extended by Tse Lok Ho who used realistic chemical species and coined the term congeneric.
[Your author has spent many hours reading this interesting paper.]
The HSAB Principle for Organic & Main Group Chemists
For our purposes – main group and organic reaction chemistry – the Pearson approach is very successful when comparing pairs of species:
- Sodium ion Na+ is harder than the silver ion Ag+
- Alkoxide ions, RO–, are harder than thioanions, RS–
- Copper(II) ion, Cu2+, is harder than copper(I) ion, Cu+
- The nitrogen anion end of the ambidentate cyanide ion, CN–, is harder than the carbon anion end, NC–
- The ambidentate enolate ion, has a hard oxyanion centre while the carbanion centre is softer and more nucleophilic.
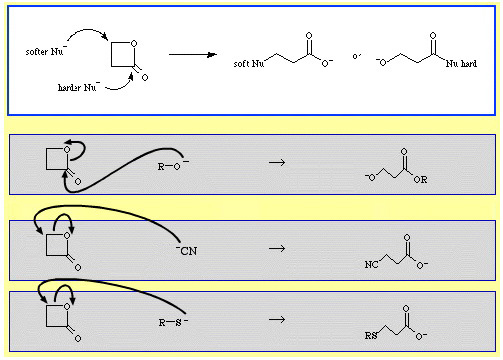
This type of analysis can be very useful in explaining reaction selectivity. For example, β-propiolactone is ring opened by nucleophilic Lewis bases. The attack can occur at two positions and nucleophiles exhibit regioselectivity:
- Harder nucleophiles like alkoxide ion, R-O–, attack the acyl (carbonyl) carbon.
- Softer nucleophiles like the cyanide ion, NC–, and the thioanion, R-S–, attack the β-alkyl carbon.
There are several examples of ambidentate selectivity in The Chemical Thesaurus reaction chemistry database:
Problems, problems, problems...
However, there are big problems with Pearson's analysis.
While the Pearson-Klopman HSAB model is not wrong... it does grossly oversimplify reaction chemistry, as recognised by Pearson.
In his 1997 book, Chemical Hardness, Wiley-VCH, pp 3-4, Pearson candidly writes:
"With [the 'Hard-Soft'] nomenclature it is possible to make a simple, general statement: 'Hard acids prefer to coordinate to hard bases, and soft acids prefer to coordinate soft bases.' This is the Principle of Hard and Soft Acids and Bases, or the HSAB Principle.
"Note that this Principle is simply a restatement of the experimental evidence which led to [the classification system in the first place]. It is a condensed statement of a very large amount of chemical information. As such it might be called a law. But this label seems pretentious in view of the lack of a quantitative definition of hardness.
"HSAB is not a theory, since it does not explain variations in the strength of chemical bonds. The word 'prefer' in the HSAB Principle implies a rather modest effect.
"Softness is not the only factor which determines the value of ΔH° in the equation:
A + :B → A:B
"There are many examples of very strong bonds between mismatched pairs, such as H2, formed from hard H+ and soft H–.
"H2O, OH– and O2– are all classified as hard bases, but there are great differences in their base strength, by any criterion." |
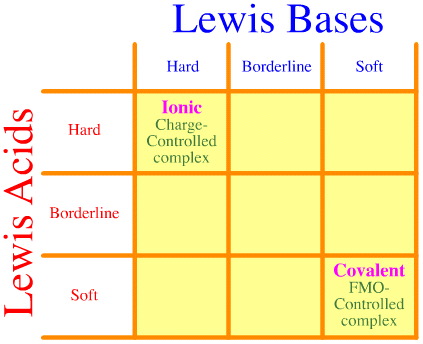
One problem is that the full set of hard-borderline-soft interactions and complexations is simply not considered using the Pearson analysis. Look how empty the HSAB interaction matrix is:
The Pearson HSAB principle states that "hard [Lewis] acids prefer to bind to hard [Lewis] bases and that soft [Lewis] acids prefer to bind to soft [Lewis] bases", which may be true, but it says nothing about mixed hard-soft complexes. Klopman simply states – very unhelpfully – that such interactions are "undefined"!
Yet, many of the most interesting reagents of organic and inorganic reaction chemistry arehard-soft "strained" complexes:
| Sodium hydride |
NaH
|
Na+
|
H–
|
| Lithium aluminium hydride |
LiAlH4
|
Al3+
|
H–
|
| Lead(IV) acetate |
Pb(AcO)4
|
Pb4+
|
AcO–
|
| Methyl lithium |
CH3Li
|
Li+
|
CH3–
|
| Triethyloxonium tetrafluoroborate |
[Et3O]+ [BF4]–
|
CH3CH2+
|
:OR2
|
| Ferrocene |
Fe(Cp)2
|
Fe2+
|
[C5H5]–
|
By comparison, the richness of known reaction chemistry arises naturally in the Lewis acid/base interaction matrix, a central tenet of the chemogenesis analysis. There are two observations/rules and both concern congeneric arrays of isoelectronic/isoreactive species:
- Hard-to-soft trends can occur within congeneric arrays, but not between arrays.
- Congeneric arrays are always found within the cells of the Lewis acid/base Interaction Matrix, and not crossing cells.
Fajans' Rules
The Pearson-Klopman HSAB analysis is in direct contradiction with the well known "Fajans rules" (1915-24) Wikipedia, even though no author appears to have addressed this issue to date.
Ionic-covalent character in metal plus non-metal binary materials can be calculated using the Pauling equation, here, but the difference in electronegativity underestimates the effect of polarisation: the extent to which one atom distorts or polarises the electron cloud of the other.
Fajans rules say:
- A small positive ion is highly polarising, favours covalency, and for a given cation the covalent character increases as the anion becomes bigger.
- Large negative ions are highly polarisible, favour covalency, and for a given anion covalent character increases as the cation gets smaller.
- Covalent character increases with increasing ionic charge on either ion.
- Polarisation, and hence covalency, is favoured if the positive ion does not have a noble gas configuration. This is important for ions like: Tl+, Pb2+, Bi3+, Ti3+, V3+, Cr2+, Mn2+, Cu+, Ce3+ & Eu2+.
Consider beryllium chloride, BeCl2: compared with the other alkaline earth chlorides:
Cation
|
Ionic
Radius
|
Eneg.
|
% Ionic of
to Cl– bond
|
Bond & Material
Type
|
Be2+
|
41
|
1.57
|
34
|
Covalent-Molecular
|
Mg2+
|
86
|
1.31
|
42
|
Ionic Salt
|
Ca2+
|
114
|
1.00
|
51
|
Ionic Salt
|
Sr2+
|
132
|
0.95
|
52
|
Ionic Salt
|
Ba2+
|
149
|
0.89
|
54
|
Ionic Salt
|
Beryllium chloride, BeCl2, is covalent: the anhydrous material is soluble in organic solvents, it sublimes (in a vacuum), and the molten material is a poor conductor of electricity. MgCl2, CaCl2, SrCl2 and BaCl2 are ionic materials.
- Fajans rules clearly explain this chemistry by saying that the very small, highly charged Be2+ ion is able to polarise the two chloride ions into a molecular covalent structure.
- The Pearson-Klopman HSAB analysis states that the beryllium ion, being the smallest of the Group II metal cations is also the hardest. Beryllium ion salts should therefore exhibit charge controlled bonding and give rise to ionic materials, but they do not.
- The chemogenesis analysis, here, says that Group II cations: Be2+, Mg2+, Ca2+, Sr2+ & Ba2+, make up a congeneric series of charged s-LUMO Lewis acids, that linear behaviour trends are found over this series. These linear behaviour trends can be ascribed to 'hard-soft' behaviour, if so wished, however, the terms 'hard' and 'soft' can only be used with respect to the congeneric series in question and 'hard-soft' comparisons cannot be made with other Lewis acids.
What's going on?
The point is that no physical parameter correlates with hardness over Pearson's chosen set of species. This creates ambiguities, such as with the organic chemistry of the fluoride ion, here, and the contradiction with Fajans rules, above.
- The Pearson model takes no account of FMO geometry (the shapes and phases of the participating orbitals). For example, just how similar are Pearson's hard Lewis acids:
H+ [NH4]+ BF3 CO2 Cs+ Cu2+ ?
Or, how similar are Pearson's soft Lewis bases:
H– R2S: H3C– benzene ?
- Crucially for organic and main group chemists, the HSAB analysis says little about the carbenium ion (carbocation) Lewis acid, H3C+, or the methyl carbanion Lewis base, H3C–.
Bold Claim
The one-dimensional hard-borderline-soft continuum of Pearson's analysis actually has the effect of blurring much of the rich, linear (predictable) behaviour that can be found in Lewis acid/base reaction chemistry space.
The new chemogenesis analysis – as presented in this web book and backed by the reaction chemistry held in The Chemical Thesaurus database – avoids and explains the pitfalls of Pearson's much hyped HSAB approach. |
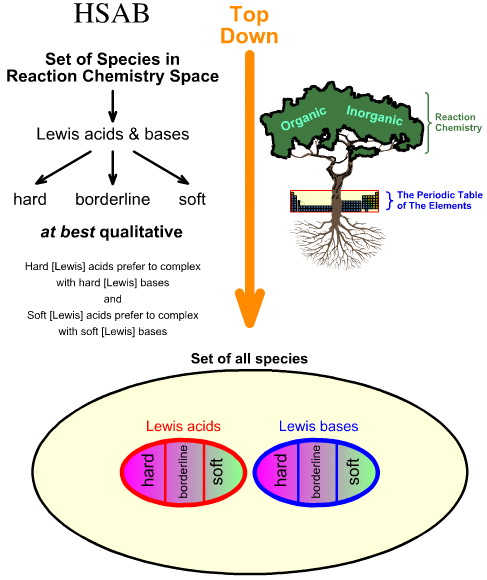
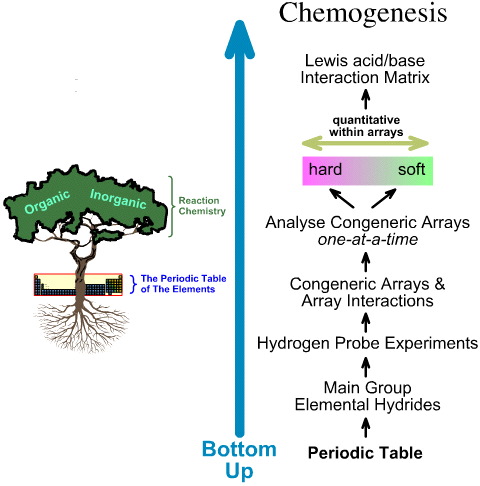
Comparing the "Top Down" HSAB Analysis with the "Bottom Up" Chemogenesis Analysis
Pearson's Hard Soft [Lewis] Acid Base (HSAB) analysis is top down.
- Starting with all species in reaction chemistry space, a number of important species are identified as Lewis acids and Lewis bases.
- Lewis acids and Lewis bases are then classified as hard, borderline or soft using empirical observation and the principle that: hard Lewis acids prefer to complex with hard Lewis bases and soft Lewis acids prefer to complex with soft Lewis bases:
The chemogenesis analysis is bottom up.
- The main group elemental hydrides are subjected to the 5 hydrogen probe experiments.
- Congeneric arrays and array interactions are studied.
- Linear hard-to-soft structural and reactivity trends are identified within arrays, and it is recognised that linear behaviour cannot expected between arrays.
- Lewis acids and Lewis bases are classified by their Lewis electronic structures and FMO topologies and are arranged into a Lewis acid/base interaction matrix, here.
The HSAB Papers:
R.G.Pearson, J.Am.Chem.Soc., 85, 3533-3543, 1963
R.G.Pearson, Science, 151, 172-177, 1966
R.G.Pearson, Chem. Br., 3, 103-107, 1967
R.G.Pearson, J.Chem.Ed., 45, 581-587, 1968
R.G.Pearson, Chemical Hardness, Wiley-VCH (1997)
G.Klopman and R.F.Hudson, Theoret. Chim. Acta, 8, 165, 1967
G.Klopman, J.Am.Chem.Soc., 90, 223-234, 1968
Also look here.
| Lewis & Brønsted Theories of Acidity |
Lewis Acids & Lewis Bases, a New Analysis
|
© Mark R. Leach 1999-2013
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,
please contact Mark R. Leach, the author, using mrl@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.
|













In acid-base catalysis, the chemical reaction is accelerated by the addition of an acid or a base, and the acid or base itself is not consumed in the reaction. Proton transfer is the commonest reaction that enzymes perform. Proton donors and acceptors, i.e. acids and base may donate and accept protons in order to stabilize developing charges in the transition state. acid base catalysis
ReplyDelete